Multiple Choice
According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO+, and NO-? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 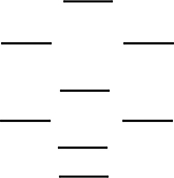
A) The bond order in NO is 2.5.
B) NO+ has the shortest bond.
C) Only one of these species is paramagnetic.
D) The bond order in NO- is 2.0.
E) NO- has the weakest bond.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which statement about VSEPR theory is not
Q96: The members of which pair of molecules
Q97: Describe the valence bond picture of bonding
Q98: How many of the following objects are
Q100: The bond angle in a trigonal planar
Q102: Which statement A-D about sigma (
Q103: Use the relative energies of the
Q104: Arrange the interactions between pairs of electrons
Q106: Identify the molecular geometry of the
Q154: Identify the polar molecule.<br>A)CF<sub>4</sub><br>B)SiH<sub>4</sub><br>C)CHCl<sub>3</sub><br>D)CS<sub>2</sub><br>E)CO<sub>2</sub>