Essay
Use the relative energies of the molecular orbitals given below, which are derived from the n = 5 atomic orbitals, to determine the bond orders in I2 ,
, and
, and identify the species that is predicted to be the most stable. The valence molecular orbitals in order of increasing energy are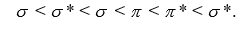
Correct Answer:

Verified
The valence electron configura...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q54: Which statement about VSEPR theory is not
Q98: How many of the following objects are
Q100: The bond angle in a trigonal planar
Q101: According to the molecular orbital energy-level diagram
Q102: Which statement A-D about sigma (
Q104: Arrange the interactions between pairs of electrons
Q106: Identify the molecular geometry of the
Q108: What is the valence electron molecular
Q154: Identify the polar molecule.<br>A)CF<sub>4</sub><br>B)SiH<sub>4</sub><br>C)CHCl<sub>3</sub><br>D)CS<sub>2</sub><br>E)CO<sub>2</sub>
Q178: Carbonyl dihalides (COX<sub>2</sub> with X = I,