Multiple Choice
An atom in its ground state absorbs a single photon of light and then relaxes back to the ground state by emitting an infrared photon (1,200 nm) followed by an orange photon (600 nm) . What is the wavelength of the photon that was absorbed initially? 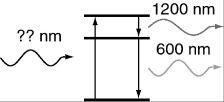
A) 600 nm
B) 1,200 nm
C) 1,800 nm
D) 900 nm
E) 400 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: Which monatomic ion most likely does not
Q53: The atomic radius of germanium (Z =
Q54: Across a row in the periodic
Q55: Which statement A-D about the wave function
Q56: If the principal quantum number is
Q58: How many orbitals exist for the
Q59: Which listing has the orbitals in order
Q60: Which of the following photons has
Q61: Which orbital has the highest energy
Q62: Which of the following photons has