Multiple Choice
Which statement A-D about the wave function for a single electron is not correct?
A) A French graduate student named Louis de Broglie first had the idea that an electron should be described as a wave.
B) Since 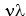 = c, an expression for the wavelength of an electron can be found by combining Einstein's equation that relates mass and energy (E = mc2) with his equation that gives the energy of a photon in terms of its frequency (E = 11ef1a67_6eab_04e9_a3cb_813dba8b61e6_TB3834_11) .
= c, an expression for the wavelength of an electron can be found by combining Einstein's equation that relates mass and energy (E = mc2) with his equation that gives the energy of a photon in terms of its frequency (E = 11ef1a67_6eab_04e9_a3cb_813dba8b61e6_TB3834_11) .
C) The square of the wave function's amplitude at a particular point in space gives the probability density of finding the electron at that point.
D) Wave functions can have nodes, which are points where the amplitude is zero.
E) Statements A-D are all correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q50: The size of a typical atomic orbital
Q51: Which transition metal ion has no d
Q52: Which monatomic ion most likely does not
Q53: The atomic radius of germanium (Z =
Q54: Across a row in the periodic
Q56: If the principal quantum number is
Q57: An atom in its ground state absorbs
Q58: How many orbitals exist for the
Q59: Which listing has the orbitals in order
Q60: Which of the following photons has