Multiple Choice
If each of the following metals is exposed to light with a wavelength of 240 nm, which will emit photoelectrons with the greatest kinetic energy?
A) iron (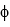 = 7.2 *10-19 J)
= 7.2 *10-19 J)
B) platinum (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 9.1 *10-19 J)
C) nickel (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 8.3 *10-19 J)
D) palladium (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 8.2 *10-19 J)
E) sodium (11ef1a66_0479_eb58_a3cb_57e06677e6de_TB3834_11 = 4.4 *10-19 J)
Correct Answer:

Verified
Correct Answer:
Verified
Q131: What is the frequency (v, in
Q132: Which of the following metals would be
Q133: How many of these atoms or ions
Q134: Copper forms a cation with a charge
Q135: Which arrangement is in the correct order
Q137: What color will a blue object appear
Q138: The ion of which element is most
Q139: Which of the following orbitals is the
Q140: Based on the following graph, which metal
Q141: What <span class="ql-formula" data-value="\ell"><span class="katex"><span