Multiple Choice
Which of the following orbitals is the lowest in energy (assuming they are all in the same shell) ?
A) 
B) 
C) 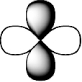
D) 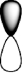
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: Which statement about electromagnetic radiation is not
Q133: How many of these atoms or ions
Q134: Copper forms a cation with a charge
Q135: Which arrangement is in the correct order
Q136: If each of the following metals is
Q137: What color will a blue object appear
Q138: The ion of which element is most
Q140: Based on the following graph, which metal
Q141: What <span class="ql-formula" data-value="\ell"><span class="katex"><span
Q143: How many unpaired electrons does the nitride