Multiple Choice
The radii of the Bohr orbits in atomic hydrogen are given by 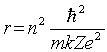 .If the radius of the first Bohr orbit n = 1) is 0.053 nm, the radius of the third Bohr orbit n = 3) is
.If the radius of the first Bohr orbit n = 1) is 0.053 nm, the radius of the third Bohr orbit n = 3) is
A) 0.16 nm.
B) 0.018 nm.
C) 0.48 nm.
D) 0.35 nm.
E) 1.3 nm.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The red line in the hydrogen emission
Q40: The radius of the n = 1
Q70: Light falling on the surface of a
Q140: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" The above figure
Q141: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" The graph shows
Q142: The energy of an x-ray photon of
Q143: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7291/.jpg" alt=" The set of
Q147: For an electron to have a de
Q149: The energy of an electron in the
Q150: Calculate the photon energy for light of