Multiple Choice
In the acid-base reaction between ammonia and water, which of the following statements best describes the concentration of ammonia and ammonium at equilibrium? 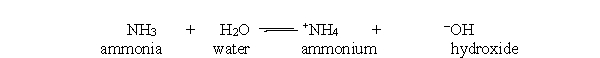
A) The concentration of ammonium is increasing.
B) There is no ammonium at equilibrium.
C) Their concentrations are equal.
D) Their concentrations are constant.
E) There is no ammonia at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following solutions has the
Q9: How do Type I and Type II
Q10: The following is the reaction of benzoic
Q12: Which of the following equations best describes
Q13: A buffer solution contains H<sub>2</sub>CO<sub>3</sub> and HCO<sub>3</sub><font
Q14: What is the pH of a solution
Q15: Which of the following atomic diagrams best
Q16: What does K<sub>a</sub> measure?<br>A) the concentration of
Q52: Which statement about neutralization reactions is FALSE?<br>A)
Q77: A weak acid is also a _