Multiple Choice
A buffer solution contains H2CO3 and HCO3, resulting in the equilibrium shown by the reaction below. According to this reaction and LeChatelier's principle, what happens when acid (H3O+) is added to this buffer solution? 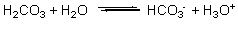
A) More HCO3 is formed.
B) More H3O+ is formed.
C) More H2CO3 is formed
D) Less H2O is formed.
E) The reaction shifts to the right.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following solutions has the
Q9: How do Type I and Type II
Q10: The following is the reaction of benzoic
Q11: In the acid-base reaction between ammonia and
Q12: Which of the following equations best describes
Q14: What is the pH of a solution
Q15: Which of the following atomic diagrams best
Q16: What does K<sub>a</sub> measure?<br>A) the concentration of
Q17: Which substance is acting as the acid
Q18: According to the Arrhenius definition, which of