Multiple Choice
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. What will happen when a small amount of base (OH) is added to the HF/F buffer? 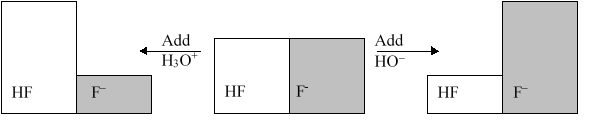
A) The concentration of -OH will increase.
B) The concentration of H3O+ will increase.
C) The concentration of HF will increase.
D) The concentration of F will increase.
E) The pH of the solution will increase.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Consider a buffer solution containing CH<sub>3</sub>COO<font face="symbol"><sup></sup></font>Na<sup>+</sup>
Q55: Adding an acid to pure water will
Q56: The following figure illustrates the action of
Q57: Lidocaine, a common injectable dental anesthetic, is
Q57: Which of the following types of molecules
Q58: In the acid-base reaction between ammonia and
Q60: Which species in the following neutralization reaction
Q61: Each circle is a sample of an
Q62: The neutralization reaction of potassium hydrogen carbonate
Q64: When a base is dissolved in water,