Multiple Choice
The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. If you add hydronium until all of the F is converted into HF and then add a little more hydronium, what is observed? 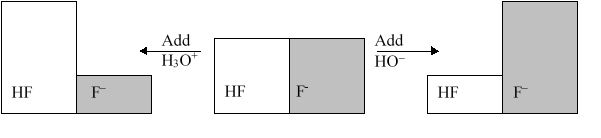
A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.
Correct Answer:

Verified
Correct Answer:
Verified
Q51: What is an enteric coating?<br>A) It is
Q52: Which of the following solutions has the
Q53: Each circle is a sample of an
Q54: Consider a buffer solution containing CH<sub>3</sub>COO<font face="symbol"><sup></sup></font>Na<sup>+</sup>
Q55: Adding an acid to pure water will
Q57: Lidocaine, a common injectable dental anesthetic, is
Q58: In the acid-base reaction between ammonia and
Q59: The following figure illustrates the action of
Q60: Which species in the following neutralization reaction
Q61: Each circle is a sample of an