Multiple Choice
In this equation 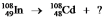 , what particle or type of radiation needs to be included on the right-hand side in order to balance it?
, what particle or type of radiation needs to be included on the right-hand side in order to balance it?
A) alpha
B) beta
C) gamma
D) positron
E) proton
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Carbon-14 will emit a <span
Q3: It is believed that two carbon-12 nuclei
Q4: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The isotope
Q6: The nuclide Pb-210 undergoes three successive decays
Q7: 11 Select the nuclide that completes the
Q9: A 55-kg person exposed to thorium-234
Q10: Which of the following isotopes is most
Q18: Exposure to 10 nCi for 10 minutes
Q55: Bombardment of uranium-238 nuclei by carbon-12 nuclei
Q72: An isotope with a low value of