Multiple Choice
Calculate S for the reaction 
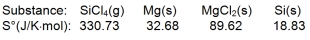
A) -254.96 J/K
B) -198.02 J/K
C) 198.02 J/K
D) 254.96 J/K
E) 471.86 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: State the second and third laws of
Q11: What is the free energy change,
Q12: Given: C<sub>2</sub>H<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q13: Which relationship or statement best describes
Q14: Given: H<sub>2</sub>O(l) <span class="ql-formula" data-value="\rightarrow"><span
Q15: The reaction of methane with water to
Q17: For a chemical reaction to be
Q19: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q20: A reaction has a positive value
Q25: The free energy of a perfect crystal