Multiple Choice
Calculate G for the combustion of propane. 
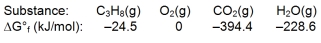
A) -2073.1 kJ
B) -1387.3 kJ
C) -598.5 kJ
D) 598.5 kJ
E) 2073.1 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: State the second and third laws of
Q14: Given: H<sub>2</sub>O(l) <span class="ql-formula" data-value="\rightarrow"><span
Q15: The reaction of methane with water to
Q16: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q17: For a chemical reaction to be
Q20: A reaction has a positive value
Q22: Use the given data at 298
Q23: Which of the following values is
Q24: "A diamond is forever" is one
Q25: The free energy of a perfect crystal