Multiple Choice
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak acid (0.10 mol L¯1) with a strong base of the same concentration?
A) 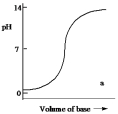
B) 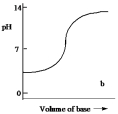
C) 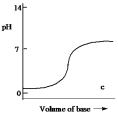
D) 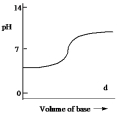
E) 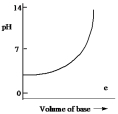
Correct Answer:

Verified
Correct Answer:
Verified
Q29: What volume of 0.200 M KOH must
Q29: Write the ion product expression for silver
Q30: Barium sulfate (BaSO<sub>4</sub>) is a slightly soluble
Q31: A 25.0-mL sample of 0.10 M C<sub>2</sub>H<sub>3</sub>NH<sub>2</sub>
Q33: A popular buffer solution consists of carbonate
Q35: The pH of blood is 7.35. It
Q39: The salts X(NO<sub>3</sub>)<sub>2</sub> and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q52: Use a carefully drawn and labeled diagram
Q89: What is the pK<sub>a</sub> for the acid
Q103: Make a clear distinction between buffer range