Multiple Choice
Write the ion product expression for silver sulfide, Ag2S.
A) 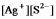
B) 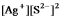
C) 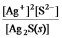
D) 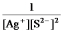
E) 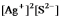
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: What is the maximum amount of sodium
Q25: A 20.0-mL sample of 0.30 M HClO
Q26: Calculate the solubility of silver oxalate, Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,
Q27: Which of the following aqueous mixtures would
Q28: A 10.0-mL sample of 0.75 M CH<sub>3</sub>CH<sub>2</sub>COOH
Q29: What volume of 0.200 M KOH must
Q30: Barium sulfate (BaSO<sub>4</sub>) is a slightly soluble
Q31: A 25.0-mL sample of 0.10 M C<sub>2</sub>H<sub>3</sub>NH<sub>2</sub>
Q33: A popular buffer solution consists of carbonate
Q34: Which one of the following is the