Multiple Choice
Calculate the solubility of zinc hydroxide, Zn(OH) 2, in 1.00 M NaOH. 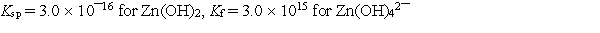
A) 0.60 M
B) 0.52 M
C) 0.37 M
D) 0.32 M
E) 0.24 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: A buffer is prepared by adding 100
Q30: What volume of 0.500 M H<sub>2</sub>SO<sub>4 </sub>is
Q44: A solution is prepared by mixing 50.0
Q45: The concentration of the complex ion in
Q46: Citric acid has an acid dissociation constant
Q47: What is the pH of a buffer
Q48: The solubility of silver chromate is 0.0287
Q51: What is the [H<sub>3</sub>O<sup>+</sup>] in a buffer
Q60: Increasing the concentrations of the components of
Q79: An acetate buffer has a pH of