Multiple Choice
The concentration of the complex ion in each of following solutions is 1.00 M. In which of the solutions will the concentration of the uncomplexed metal ion be the greatest? 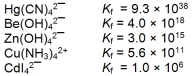
A) Hg2+
B) Be2+
C) Zn2+
D) Cu2+
E) Cd2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: A buffer is prepared by adding 0.5
Q41: Calculate the solubility of magnesium sulfate, MgSO<sub>4</sub>,
Q42: Which one of the following is the
Q44: A solution is prepared by mixing 50.0
Q46: Citric acid has an acid dissociation constant
Q47: What is the pH of a buffer
Q48: The solubility of silver chromate is 0.0287
Q49: Calculate the solubility of zinc hydroxide, Zn(OH)<sub>2</sub>,
Q60: Increasing the concentrations of the components of
Q105: A 50.0-mL sample of 0.50 M HCl