Multiple Choice
The equilibrium constant Kc for the reaction 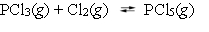 is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
A) 0.049 M
B) 0.11 M
C) 0.30 M
D) 0.59 M
E) 0.83 M
Correct Answer:

Verified
Correct Answer:
Verified
Q16: For a gas-phase equilibrium, a change in
Q61: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.
Q75: At 850 <span class="ql-formula" data-value="\degree"><span
Q76: Nitric oxide and bromine were allowed to
Q78: Select the mass-action expression, Q<sub>c</sub>, for the
Q79: Stearic acid, nature's most common fatty
Q81: What is the mass-action expression, Q<sub>c</sub>, for
Q82: A container was charged with hydrogen,
Q83: Unless <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q85: Given this equilibrium constant data at