Multiple Choice
Stearic acid, nature's most common fatty acid, dimerizes when dissolved in hexane: 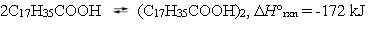 The equilibrium constant for this reaction at 28 C is 2900. Estimate the equilibrium constant at 38 C.
The equilibrium constant for this reaction at 28 C is 2900. Estimate the equilibrium constant at 38 C.
A) 4.7 * 105
B) 2.6 * 104
C) 1.9 * 103
D) 3.2 * 102
E) 18
Correct Answer:

Verified
Correct Answer:
Verified
Q16: For a gas-phase equilibrium, a change in
Q61: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.
Q74: Consider the following two equilibria and their
Q75: At 850 <span class="ql-formula" data-value="\degree"><span
Q76: Nitric oxide and bromine were allowed to
Q78: Select the mass-action expression, Q<sub>c</sub>, for the
Q80: The equilibrium constant K<sub>c</sub> for the
Q81: What is the mass-action expression, Q<sub>c</sub>, for
Q82: A container was charged with hydrogen,
Q83: Unless <span class="ql-formula" data-value="\Delta"><span class="katex"><span