Multiple Choice
The following reaction, in CCl4 solvent, has been studied at 25 C. 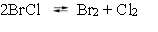
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
A) 1.35 *10¯3 M
B) 2.70 * 10¯3 M
C) 8.82 * 10¯3 M
D) 9.70 * 10¯2 M
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: For a gas-phase equilibrium, a change in
Q18: Methanol can be synthesized by combining carbon
Q19: Consider the reactions of cadmium with the
Q20: Write the mass-action expression, Q<sub>c</sub>, for the
Q23: Consider the equilibrium:<br>A(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="
Q25: 10.0 mL of a 0.100 mol L¯<sup>1</sup>
Q46: Changing the amount of a solid reactant
Q63: Once a reaction system reaches equilibrium, the
Q83: A chemical reaction will reach equilibrium when
Q85: If Q > K, more products need