Multiple Choice
10.0 mL of a 0.100 mol L¯1 solution of a metal ion M2+ is mixed with 10.0 mL of a 0.100 mol l¯1 solution of a substance L. The following equilibrium is established: 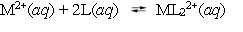 At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
A) 0.100 mol L¯1
B) 0.050 mol L¯1
C) 0.025 mol L¯1
D) 0.0200 mol L¯1
E) 0.0100 mol L¯1
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Write the mass-action expression, Q<sub>c</sub>, for the
Q22: The following reaction, in CCl<sub>4</sub> solvent,
Q23: Consider the equilibrium:<br>A(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="
Q28: Hydrogen bromide will dissociate into hydrogen
Q29: Compounds A, B, and C react
Q46: Changing the amount of a solid reactant
Q63: Once a reaction system reaches equilibrium, the
Q78: If all the reactants and products in
Q83: A chemical reaction will reach equilibrium when
Q85: If Q > K, more products need