Multiple Choice
The reaction system 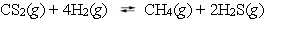 is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
A) As equilibrium is reestablished, the partial pressure of carbon disulfide increases.
B) As equilibrium is reestablished, the partial pressure of methane, CH4, decreases.
C) As equilibrium is reestablished, the partial pressure of hydrogen decreases.
D) As equilibrium is reestablished, the partial pressure of hydrogen sulfide decreases.
E) As equilibrium is reestablished, all the partial pressures will decrease.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Hydrogen iodide, HI, is formed in an
Q66: A chemical reaction has an equilibrium constant
Q67: Ammonia is synthesized in the Haber process:
Q68: The reaction quotient, Q<sub>c</sub>, for a reaction
Q69: The equilibrium constant K<sub>c</sub> for the
Q70: The reaction quotient for a gas phase
Q73: At 450 <span class="ql-formula" data-value="\degree"><span
Q74: Consider the following two equilibria and their
Q75: At 850 <span class="ql-formula" data-value="\degree"><span
Q76: Nitric oxide and bromine were allowed to