Multiple Choice
At 450 C, tert-butyl alcohol decomposes into water and isobutene. 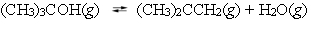 A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A) The forward reaction will proceed to reestablish equilibrium.
B) The reverse reaction will proceed to reestablish equilibrium.
C) No change occurs.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Hydrogen iodide, HI, is formed in an
Q16: For a gas-phase equilibrium, a change in
Q68: The reaction quotient, Q<sub>c</sub>, for a reaction
Q69: The equilibrium constant K<sub>c</sub> for the
Q70: The reaction quotient for a gas phase
Q71: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q74: Consider the following two equilibria and their
Q75: At 850 <span class="ql-formula" data-value="\degree"><span
Q76: Nitric oxide and bromine were allowed to
Q78: Select the mass-action expression, Q<sub>c</sub>, for the