Multiple Choice
Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. 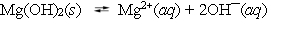 The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
A) The hydroxide ion concentration will decrease.
B) The hydroxide ion concentration will increase.
C) The hydroxide ion concentration will be unchanged.
D) The solution will become supersaturated.
E) None of these conclusions is justified without additional information.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Ethane can be formed by reacting acetylene
Q41: Write the mass-action expression, Q<sub>c</sub> , for
Q42: Sodium hydrogen carbonate decomposes above 110
Q43: At a certain temperature the reaction CO<sub>2</sub>(g)
Q44: The equilibrium constant, K<sub>p</sub>, for the
Q46: At 25 <span class="ql-formula" data-value="\degree"><span
Q48: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q49: The following reaction is at equilibrium in
Q50: Write the mass-action expression, Q<sub>c</sub>, for the
Q88: In a chemical reaction, if the starting