Multiple Choice
Ethane can be formed by reacting acetylene with hydrogen. 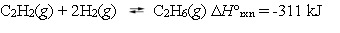 Under which reaction conditions would you expect to have the greatest equilibrium yield of ethane?
Under which reaction conditions would you expect to have the greatest equilibrium yield of ethane?
A) high temperature, high pressure
B) low temperature, high pressure
C) high temperature, low pressure
D) low temperature, low pressure
E) None of these choices is correct, unless a catalyst is present.
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Magnesium carbonate dissociates to magnesium oxide
Q36: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q37: A mixture of 0.600 mol of
Q38: Write the expressions for K<sub>c</sub> and K<sub>p</sub>
Q39: The following reaction is at equilibrium in
Q41: Write the mass-action expression, Q<sub>c</sub> , for
Q42: Sodium hydrogen carbonate decomposes above 110
Q43: At a certain temperature the reaction CO<sub>2</sub>(g)
Q44: The equilibrium constant, K<sub>p</sub>, for the
Q45: Magnesium hydroxide is used in several