Hydrogen Bromide Will Dissociate into Hydrogen and Bromine Gases C Have on This System at Equilibrium?
A) the Partial
Multiple Choice
Hydrogen bromide will dissociate into hydrogen and bromine gases. 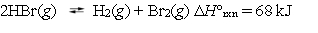 What effect will a temperature increase of 50 C have on this system at equilibrium?
What effect will a temperature increase of 50 C have on this system at equilibrium?
A) The partial pressure of hydrogen bromide will increase.
B) The partial pressure of hydrogen will increase.
C) The partial pressure of hydrogen bromide and bromine will increase.
D) There will be no effect on the partial pressure of any of the gases.
E) Need to know the initial pressure, volume and temperature before any of the above predictions can be made.
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Consider the equilibrium:<br>A(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="
Q25: 10.0 mL of a 0.100 mol L¯<sup>1</sup>
Q29: Compounds A, B, and C react
Q31: Write the mass-action expression, Q<sub>c</sub>, for the
Q32: The reaction of nitrogen with oxygen
Q33: The following reaction is at equilibrium at
Q46: Changing the amount of a solid reactant
Q78: If all the reactants and products in
Q83: A chemical reaction will reach equilibrium when
Q85: If Q > K, more products need