Multiple Choice
A study of the decomposition reaction 3RS2 3R + 6S yields the initial rate data below. What is the rate constant for the reaction? 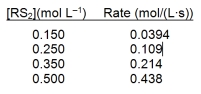
A) 0.0103 L mol¯1s¯1
B) 0.263 L mol¯1s¯1
C) 0.571 L mol¯1s¯1
D) 1.17 L mol¯1s¯1
E) 1.75 L mol¯1s¯1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: A catalyst accelerates a reaction because<br>A)it increases
Q7: A reaction has an activation energy
Q9: You are studying the rate of
Q10: Sulfur trioxide can undergo decomposition according
Q13: In the gas phase at 500.
Q16: The rate law for the reaction 3A
Q23: Reaction intermediates differ from activated complexes in
Q26: The half-life of a second-order reaction does
Q46: When the reaction A <font face="symbol"></font> B
Q56: The units of the rate constant depend