Essay
In the gas phase at 500. C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 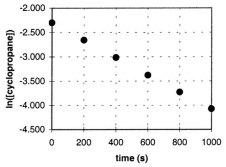 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.
B) Calculate the first-order rate constant, k.
C) Determine the initial concentration of cyclopropane in this experiment.
Correct Answer:

Verified
a. The fact that a plot of ln ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q8: A study of the decomposition reaction
Q9: You are studying the rate of
Q10: Sulfur trioxide can undergo decomposition according
Q16: The rate law for the reaction 3A
Q17: Consider the general gas-phase reaction of a
Q19: In a reversible reaction, a catalyst will
Q20: Sucrose decomposes to fructose and glucose in
Q50: An increase in temperature increases the reaction
Q56: The units of the rate constant depend
Q57: The reaction X <font face="symbol"></font> Y is