Essay
Consider the general gas-phase reaction of a molecular substance, A:
1. 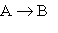 At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:
2. 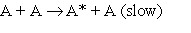 3.
3. 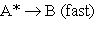 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).
Correct Answer:

Verified
a. Reactions 2 and 3 are elementary. b. ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q13: In the gas phase at 500.
Q16: The rate law for the reaction 3A
Q19: In a reversible reaction, a catalyst will
Q20: Sucrose decomposes to fructose and glucose in
Q20: When a catalyst is added to a
Q23: Tetrafluoroethylene, C<sub>2</sub>F<sub>4</sub>, can be converted to octafluorocyclobutane
Q50: An increase in temperature increases the reaction
Q53: All second-order reactions are bimolecular reactions.
Q54: According to the collision theory of reaction
Q57: The reaction X <font face="symbol"></font> Y is