Multiple Choice
Boron nitride is being investigated in frontier research directed at producing novel electronic devices.If you used the following energy-level diagram for the molecular orbitals of boron nitride, BN, what would you predict? 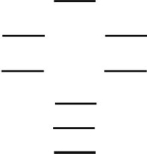
I.Boron nitride is diamagnetic.
II.Boron nitride has a bond order of 2.
III.Boron nitride is paramagnetic.
IV.The bond in BN- is weaker than the bond in BN.
A) I and II
B) II and III
C) I, II, and IV
D) III
E) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q91: What are the ideal bond angles
Q93: Which statement below regarding molecular orbitals
Q94: Which statement regarding a <span
Q95: Which statement below regarding molecular orbital
Q97: What type of hybridization is needed to
Q98: Draw the molecular orbital diagram for HCl.Use
Q99: Answer the following questions about the
Q100: What are the electron group number and
Q101: Identify the hybridization of atomic orbitals for
Q104: All homonuclear diatomic molecules _<br>A)have polar bonds.<br>B)are