Essay
Answer the following questions about the bonding in dimethyl formamide.The Lewis structure without nonbonding electrons is shown below. 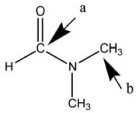 Give the following information for atom a and for atom b:
Give the following information for atom a and for atom b:
How many electron groups are present, and what is the electron-pair geometry? What is the shape associated with this bonding arrangement?
What is its hybridization?
How many and how many bonds has it formed?
Correct Answer:

Verified
Letter a has three electron groups, trig...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q94: Which statement regarding a <span
Q95: Which statement below regarding molecular orbital
Q96: Boron nitride is being investigated in frontier
Q97: What type of hybridization is needed to
Q98: Draw the molecular orbital diagram for HCl.Use
Q100: What are the electron group number and
Q101: Identify the hybridization of atomic orbitals for
Q102: Which molecule or ion below has the
Q103: What is the hybridization of the iodine
Q104: Use MO theory to predict the bond