Essay
Identify the hybridization of the atomic orbitals on carbon atoms labeled 1, 2, and 3.The hydrogen atoms and lone pair electrons are not shown in the diagram. 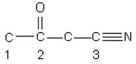
Correct Answer:

Verified
C1 is sp3, ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: What is the hybridization of selenium in
Q43: Carbonyl dihalides (COX<sub>2</sub> with X = I,
Q44: Identify the hybridization of atomic orbitals for
Q45: Which item below is achiral superimposable on
Q47: Which molecule or ion below is linear?<br>A)H<sub>2</sub>Se<br>B)H<sub>2</sub>S<br>C)ICl<sub>3</sub><br>D)IBr
Q48: Arrange the interactions between pairs of
Q49: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH.The end carbon
Q50: Which statement below best describes the
Q51: According to the molecular orbital energy-level diagram
Q183: Which type of molecular orbital is used