Essay
Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below.Explain why you think this molecule is planar or nonplanar. 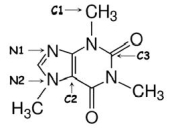
Correct Answer:

Verified
N1 is sp2, N2 is sp3, C1 is sp3, and C2 and ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q39: Consider the structure of glyceraldehyde (nonbonding
Q40: Determine the molecular geometry of the chlorite
Q41: What is the hybridization of selenium in
Q43: Carbonyl dihalides (COX<sub>2</sub> with X = I,
Q45: Which item below is achiral superimposable on
Q46: Identify the hybridization of the atomic orbitals
Q47: Which molecule or ion below is linear?<br>A)H<sub>2</sub>Se<br>B)H<sub>2</sub>S<br>C)ICl<sub>3</sub><br>D)IBr
Q48: Arrange the interactions between pairs of
Q49: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH.The end carbon
Q183: Which type of molecular orbital is used