Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of five different pure substances are shown in the figure below.Which substance has the lowest melting point? 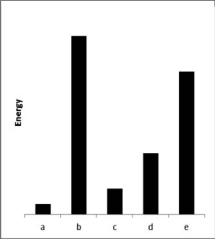
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Which compound below is capable of hydrogen
Q45: Point d in the phase diagram below
Q47: The dipole moments of HCl and of
Q47: Dispersion forces are due to _<br>A)permanent dipoles.<br>B)temporary
Q48: Which liquid below will have the highest
Q51: You decide to experiment with carbon disulfide
Q52: Which of the following ions would you
Q53: Identify the dominant intermolecular interaction or interactions
Q54: Why does HI boil at a higher
Q82: A phase diagram shows the states of