Multiple Choice
Point d in the phase diagram below is the _______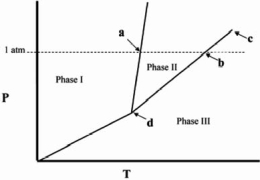
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Would water rise to the same height
Q41: Dipole-dipole interactions typically are not as strong
Q43: Viscosity is a measure of a substance's<br>A)ability
Q44: Which compound below is capable of hydrogen
Q47: The dipole moments of HCl and of
Q47: Dispersion forces are due to _<br>A)permanent dipoles.<br>B)temporary
Q48: Which liquid below will have the highest
Q49: The relative energies (strengths) of the intermolecular
Q82: A phase diagram shows the states of
Q138: Why do the strengths of dispersion interactions