Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below.Which substance is most likely to be a gas at room temperature? 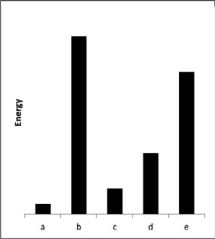
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Q72: Ion-dipole forces always require_<br>A)an ion and a
Q77: The solubility of a compound may depend
Q79: Arrange the following molecules in order of
Q80: Difluoromethane (CH<sub>2</sub>F<sub>2</sub>) has a dipole moment
Q81: Which choice below is not equal to
Q82: Which pair of compounds below is most
Q83: Which statement below is true?<br>A)Nonpolar molecules can
Q84: Which compound below would you predict can
Q85: Stearic acid, a fatty acid commonly found
Q86: Which straight-chain alkane below would you predict