Multiple Choice
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL.The sharp rise is at 10.0 mL. 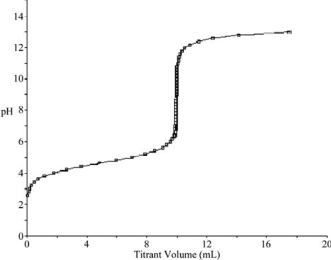
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Glycolic acid, which is a monoprotic acid
Q52: What is the pH of a solution
Q54: Suppose a 1.0 L solution containing 0.20
Q55: Which of the following species is a
Q56: The solubility product for Ag<sub>3</sub>PO<sub>4</sub> is written
Q58: Identify the Lewis base in the
Q59: A 25.00 mL sample of a phosphoric
Q61: Bromocresol green is yellow in its acidic
Q61: What would happen to the Ag<sup>+</sup> and
Q62: You are working on a project to