Multiple Choice
You are working on a project to recycle nickel and cadmium from old nickel-cadmium batteries that have an iron casing.The batteries are dissolved in aqueous nitric acid, producing a solution containing primarily Ni2+, Cd2+, and Fe3+ cations.One idea is to add sodium hydroxide to neutralize the acid and cause precipitation of Ni(OH) 2, Cd(OH) 2, and Fe(OH) 3.Assume the concentration of each of the cations is 0.100 M before the sodium hydroxide is added.The pH increases as the sodium hydroxide is added.Which compound will precipitate first, and what is the pH at that point? 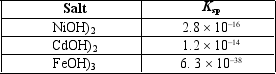
A) Cd(OH) 2; pH = 9.16
B) Fe(OH) 3; pH = 4.81
C) Fe(OH) 3; pH = 1.93
D) Cd(OH) 2; pH= 6.42
E) Ni(OH) 2; pH =7.24
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Purveyors of salts from the Dead Sea
Q57: At what point in the following titration
Q58: Identify the Lewis base in the
Q59: A 25.00 mL sample of a phosphoric
Q61: What would happen to the Ag<sup>+</sup> and
Q61: Bromocresol green is yellow in its acidic
Q63: As the pH decreases, the solubility of
Q64: How many moles of sodium acetate must
Q65: K<sub>sp</sub> for lead iodide is 8.5 *10<sup>-9</sup>
Q67: Consider the following aqueous equilibrium for a