Multiple Choice
What is indicated by the shape of the titration curve? 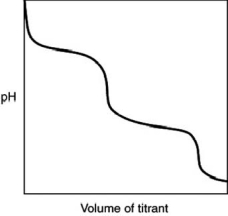
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A dibasic base was titrated with a strong acid.
D) A tribasic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Which combination of solutions is the best
Q26: The pK<sub>a</sub> of hydrated iron(III) is 2.5,
Q93: What is the pH of a solution
Q94: Write the equation for the Lewis acid-base
Q95: What is true about the pK<sub>a</sub> of
Q96: Glycolic acid, which is a monoprotic acid
Q97: A 0.500 g sample of an unknown
Q99: Write the equation for the Lewis acid-base
Q101: Calculate the pH of a solution that
Q102: Which solution below would be the best