Multiple Choice
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution.Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution.The resulting titration curves are illustrated here.Given the following possibilities, what is the sample? 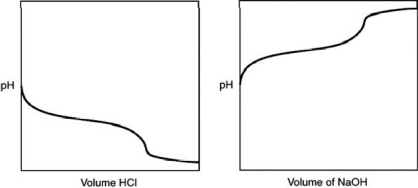
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Which combination of solutions is the best
Q92: The solubility product for PbCl<sub>2</sub> is written
Q93: What is the pH of a solution
Q94: Write the equation for the Lewis acid-base
Q95: What is true about the pK<sub>a</sub> of
Q96: Glycolic acid, which is a monoprotic acid
Q98: What is indicated by the shape of
Q99: Write the equation for the Lewis acid-base
Q101: Calculate the pH of a solution that
Q102: Which solution below would be the best