Multiple Choice
Use the table of standard reduction potentials below to identify the species that is the strongest reducing agent. 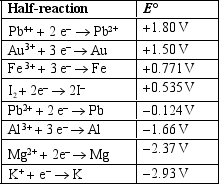
A) Pb4+
B) PB2+
C) K+
D) K
E) Al
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q112: Based on the cell diagram, Fe(s) |
Q113: If the potential of a voltaic cell
Q114: Which metal would NOT be a good
Q115: In the smelting of iron from
Q116: The magnitude of the charge on a
Q118: The oxidation of hydrogen by oxygen
Q119: Methanol fuel cells depend on the
Q120: Neuron cells generate electrical signals by concentration
Q121: How long would it take to electroplate
Q122: Based on the information in the table