Multiple Choice
Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron Fe) and magnesium Mg) electrodes immersed in 1M Fe3+and Mg2+solutions? Also, identify the cathode. 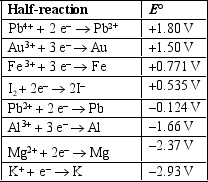
A) (+1.60 V with Fe as the cathode)
B) (-1.60 V with Mg as the cathode)
C) (-3.14 V with Fe as the cathode)
D) (-3.14 V with Mg as the cathode)
E) (+3.14 V with Fe as the cathode)
Correct Answer:

Verified
Correct Answer:
Verified
Q117: Use the table of standard reduction potentials
Q118: The oxidation of hydrogen by oxygen
Q119: Methanol fuel cells depend on the
Q120: Neuron cells generate electrical signals by concentration
Q121: How long would it take to electroplate
Q123: Which statement below regarding chemical energy and
Q124: In one episode of the 1960s television
Q125: The standard hydrogen electrode is<br>A)used to calibrate
Q126: Reduction refers to<br>A)loss of mass.<br>B)an increase in
Q127: Using the following data, determine E<sup>o</sup><sub>cell