Multiple Choice
In the figure shown, what does the dotted line represent? 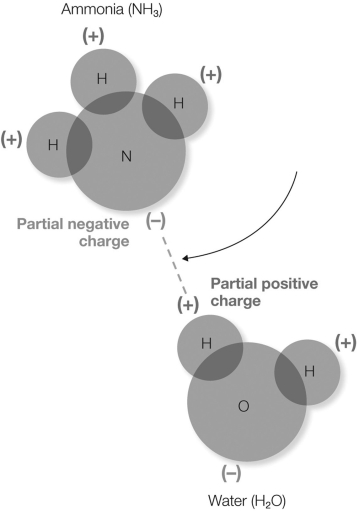
A) transfer of the electron from the hydrogen atom to the nitrogen atom
B) sharing of electrons between the ammonia and water molecules
C) a Van der Waals interaction
D) an electrostatic interaction between the partially- positive hydrogen and the partially- negative nitrogen
E) an interaction between a hydrophilic and a hydrophobic molecule
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Which of the following is not one
Q26: The figure shown is an exergonic reaction
Q27: A cation forms when an atom loses
Q28: In the human genetic disease sickle cell
Q29: What information can you determine about the
Q31: Acids increase the H+ concentration in a
Q32: Which of the biomolecules is incorrectly matched
Q33: In a polar covalent bond,<br>A) electrons are
Q34: Hydrogen bonds<br>A) form whenever hydrogen is involved
Q35: A phosphodiester bond links a fatty acid