Multiple Choice
The figure shown is an exergonic reaction because 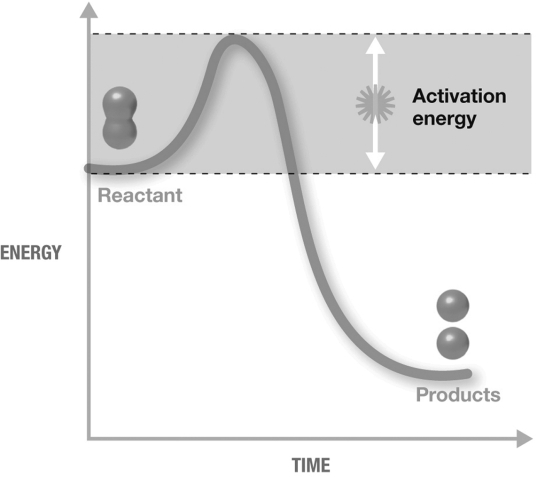
A) activation energy was required.
B) it is a decomposition reaction and the products have a lower final energy than the reactants.
C) it is a decomposition reaction.
D) the products have a lower final energy than the reactants.
E) activation energy was required and the products have a lower final energy than the reactants.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Describe the relationship between acids, bases, salts,
Q22: Plasma membranes must be in a fluid
Q23: Water (H<sub>2</sub>O) and carbon dioxide (CO<sub>2</sub>) are
Q24: A molecule of glucose contains six carbon
Q25: Which of the following is not one
Q27: A cation forms when an atom loses
Q28: In the human genetic disease sickle cell
Q29: What information can you determine about the
Q30: In the figure shown, what does the
Q31: Acids increase the H+ concentration in a