Multiple Choice
How much energy is required to remove a neutron (mn = 1.008 665 u) from 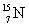 that has an atomic mass of 15.000 108 u to make
that has an atomic mass of 15.000 108 u to make 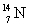 that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
that has an atomic mass of 14.003 074 u? Note: The energy equivalent of the atomic mass unit is 931.5 MeV.
A) 1.163 MeV
B) 6.423 MeV
C) 10.83 MeV
D) 928.7 MeV
E) 939.6 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which one of the following statements concerning
Q2: Determine the amount of energy released in
Q4: How many neutrons are there in the
Q4: The half-life a particular isotope of barium
Q6: This question refers to the figure shown.Which
Q8: What is the SI unit for activity?<br>A)Ci<br>B)counts/min<br>C)Hz<br>D)Gy<br>E)Bq
Q10: The half-life of a particular isotope of
Q24: Determine the activity of 6.0 × 10<sup>12</sup>
Q32: The activity of a carbon-14 sample is
Q36: The binding energy of an isotope of