Multiple Choice
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents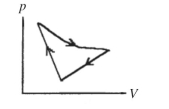
A) the heat added to the gas.
B) the work done by the gas.
C) the heat that flows out of the gas.
D) the work done on the gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A certain heat engine extracts
Q6: One of the most efficient engines
Q7: An ideal gas undergoes the process
Q8: A certain gas is compressed adiabatically.
Q9: A gas expands from an initial
Q11: What is the change in entropy
Q12: When <span class="ql-formula" data-value="0.50 \mathrm
Q13: The ocean thermal energy conversion project
Q14: A monatomic ideal gas undergoes an
Q15: During each cycle, a refrigerator removes