Multiple Choice
An ideal gas undergoes the process shown in the diagram. In this figure, , and . How much work is done by the system in this process?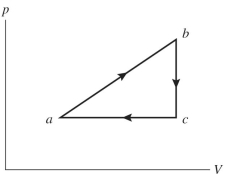
A)
B)
C)
D)
E)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: A heat engine with an efficiency
Q3: A container of ideal gas at
Q4: What is the efficiency of an
Q5: A certain heat engine extracts
Q6: One of the most efficient engines
Q8: A certain gas is compressed adiabatically.
Q9: A gas expands from an initial
Q10: A cyclic process is carried out
Q11: What is the change in entropy
Q12: When <span class="ql-formula" data-value="0.50 \mathrm