Multiple Choice
The figure shows a diagram for of ideal nitrogen gas in a sealed container. The temperature of state 1 is , the atomic mass of the nitrogen atom is , and - K. What are (a) pressure and (b) temperature ?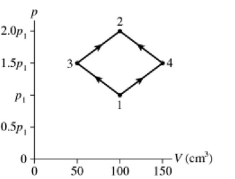
A) (a) , (b)
B) (a) 81 atm, (b)
C) (a) , (b)
D) (a) , (b)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Two processes are shown on the
Q58: The figure shows a <span
Q59: A heat engine receives <span
Q60: The figure shows a <span
Q61: If the efficiency of a Carnot
Q63: A 40.0-L container is divided into
Q64: What is the change in entropy
Q65: An ideal gas undergoes the process
Q66: On a cold winter day, the
Q67: A heat engine has an efficiency of