Multiple Choice
An ideal gas undergoes the process shown in the diagram. The heat gained by the gas in process is , while in process the gas loses of heat. In process the gas performs of work, while in process of work is done on the gas. How much heat is gained by the gas in process ?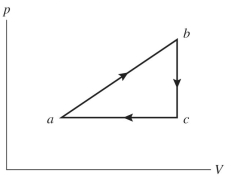
A)
B)
C)
D)
E)
Correct Answer:

Verified
Correct Answer:
Verified
Q29: A heat engine has an efficiency of
Q30: An ideal Carnot engine operating between
Q31: What is the entropy change of
Q32: The figure shows a <span
Q33: For a certain ideal Carnot engine,
Q35: Two ideal Carnot heat engines have
Q36: A compression at a constant pressure
Q37: The figure shows a <span
Q38: A certain automobile engine takes in 4.00
Q39: A Carnot cycle consists of<br>A) four isotherms.<br>B)